Be sure to catch up on our latest webinar hosted by Dr. Michael Sy to explore the MSDA Test in a clinical setting. Watch now.
FOR PROVIDERS
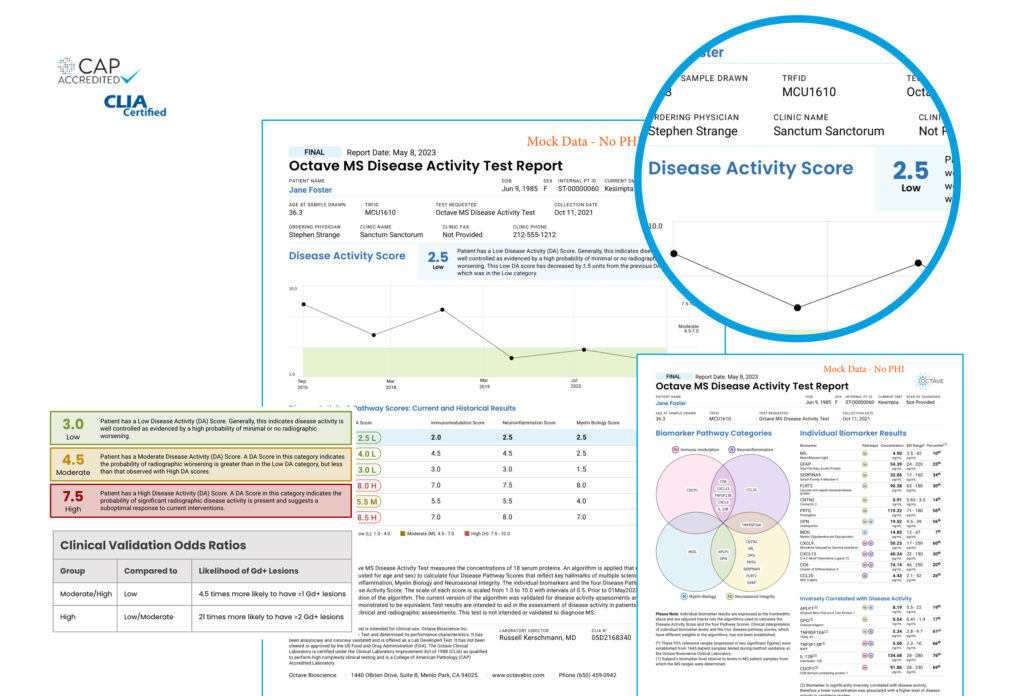
With precise, personalized results, the Octave MSDA test provides clinical data to support informed care decisions.

Across all clinical scenarios, clinicians need a starting point. The MSDA Test delivers a measurable baseline to inform and track care over time.




answer
answer
answer
answer
Speak with our clinical support team to learn more about implementing the MSDA Test.
