New real-world evidence on the MSDA Test published in The Multiple Sclerosis Journal – ET&C. Read the press release.
CLINICAL EVIDENCE & UTILITY
Real-world evidence, as published in Multiple Sclerosis Journal – Experimental, Translational and Clinical, confirms MSDA’s impact on guiding treatment decisions.

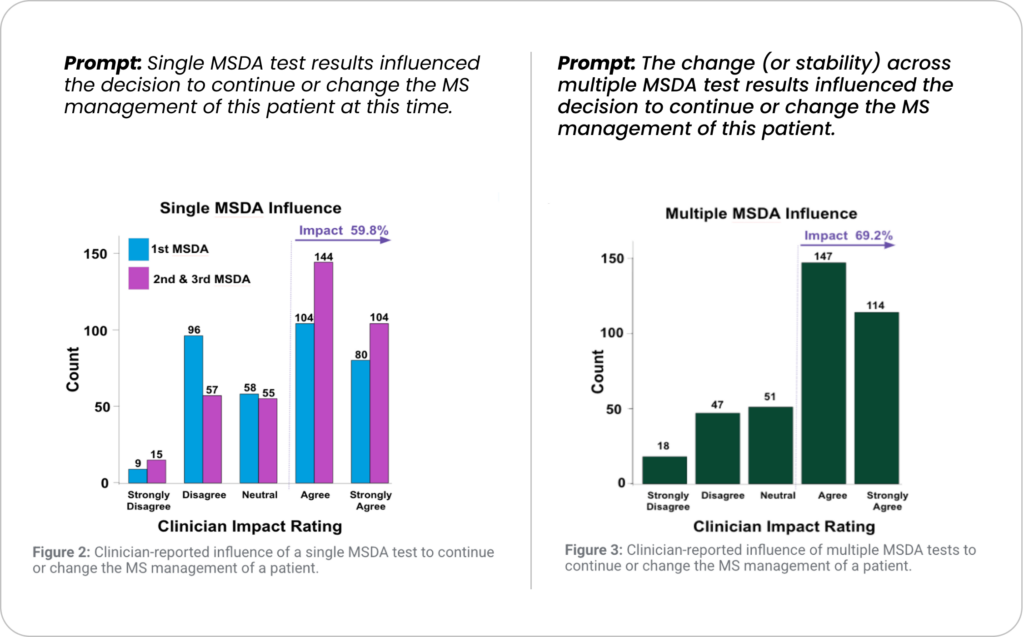
How influential is the MSDA Test to clinical decision making in the opinion of the clinician?
The MSDA Test significantly impacts decisions at meaningful decision timepoints.
With continued use of the test, the MSDA aided in the clinicians’ decisions to start, stop or switch DMT and/or order additional lab or MRI tests.
A total of 352 charts which included 723 MSDA tests were reviewed. The proportion of decision time points where clinicians “strongly agreed” or “agreed” that MSDA results influenced their decision-making was greater when multiple longitudinal MSDA results were available compared to a single MSDA result: 69.2% (p<0.001; 95%CI: [60.2, 78.3]%) vs. 59.8% (p=0.217; 95%CI: [43.7, 76]%), respectively (FIGS. 2 & 3).

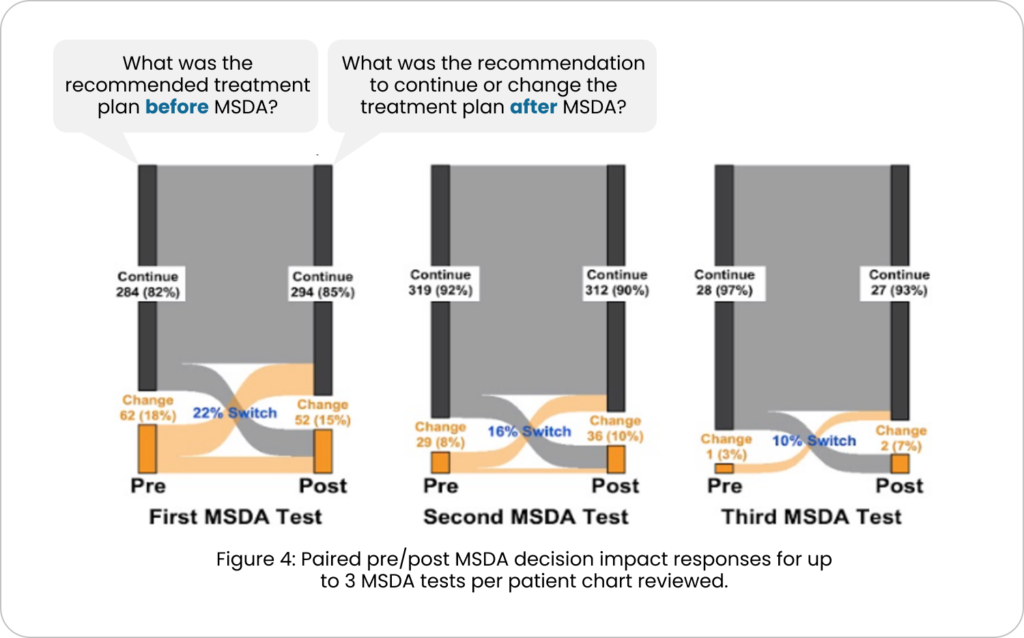
Does the clinician change clinical decisions on the basis of The MSDA Test?
Evidence shows particular value in longitudinal monitoring.
Pre-post MSDA chart review captured clinical recommendation(s) before and after MSDA results were received. The clinical decision to be made were to continue or change current plan, including starting, stopping or switching DMTs, prescribing steroids or ordering additional labs/MRU based on results of the MSDA Test.
The overall rate of clinical decision changes after MSDA testing across all decision time points (first, second, and third MSDA tests) (FIG. 4) was 19.4%.

What MSDA results prompt which changes in clinical decisions?
Demonstrates utility through objective treatment plan changes.
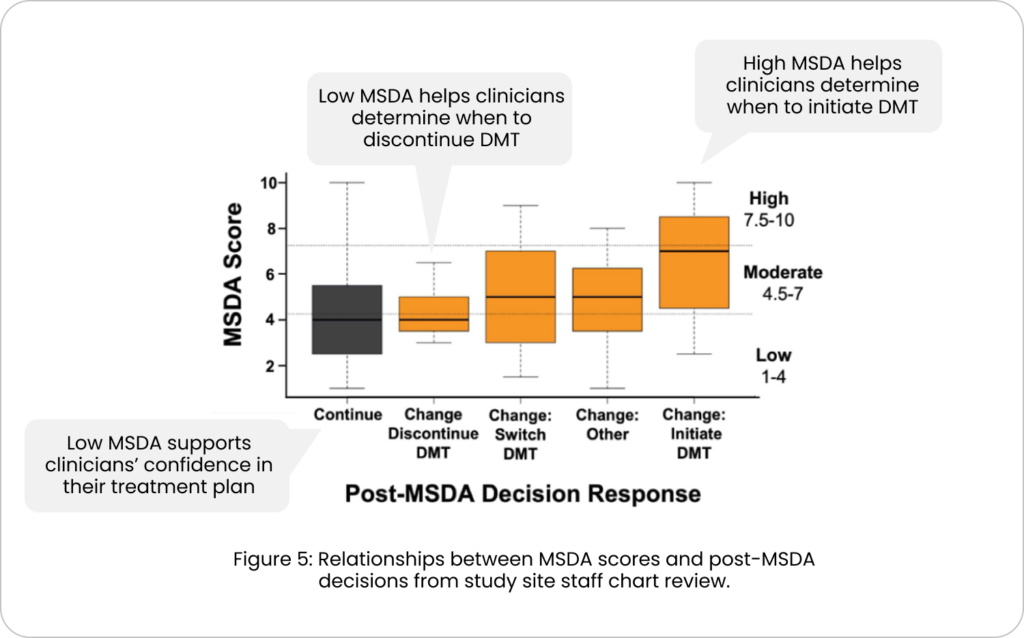
Three patterns in clinician MS management were observed following receipt of MSDA results : 1) A low MSDA score supported the clinician’s plan to continue the current treatment plan, 2) A low MSDA score helped determine when a patient could discontinue a DMT, and 3) A high MSDA score identified when a patient should initiate a DMT
(FIG. 5).
Treatment change trends were most common among low & high MSDA scores, instilling confidence in staying the course and DMT stop, start and switch.